Build an Atom - PhET Interactive Simulations.
- In 1913 Neils Bohr proposed his model of atom which superceded Rutherford's atomic model. Though the planetary model proposed by Rutherford was widely accepted, it fell short on many counts. The nuclear atom proposed by Rutherford was unstable. According to classical theories this atom should collapse.
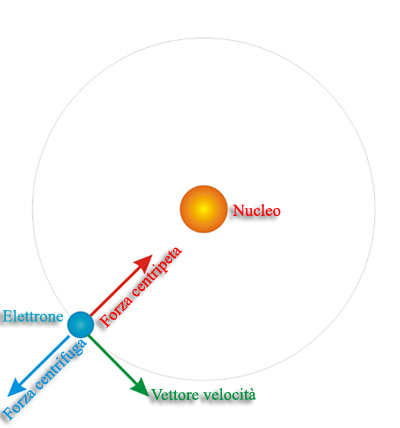
- Bohr agreed with Rutherford’s proposal that in the atom the electrons revolve around a central positively charged nucleus that is responsible for most of the weight of the atom. But from his special evidence, he concluded that electrons are found at only certain distances from the.
| The Bohr Model |
The most important properties of atomic and molecular structure may beexemplified using a simplified picture of an atom that is called the BohrModel. This model was proposed by Niels Bohr in 1915; itis not completely correct, butit has many features that are approximately correctand it is sufficient for much of our discussion. The correct theory of theatom is called quantum mechanics; the Bohr Model is an approximationto quantum mechanics that has the virtue of being much simpler.(Here is a more realistic discussion of what atomic orbitals look like in quantummechanics.)


A Planetary Model of the Atom
| The Bohr atom |

This similarity between a planetary model and the Bohr Model of the atomultimately arises because the attractivegravitational force in a solar systemand the attractiveCoulomb (electrical) force between the positively charged nucleus andthe negatively charged electrons in an atom are mathematically of the same form.(The form is the same, but the intrinsicstrength of the Coulombinteraction is much larger than that of the gravitational interaction; inaddition, there are positive and negative electrical charges so the Coulombinteraction can be either attractive or repulsive, but gravitation is alwaysattractive in our present Universe.)

But the Orbits Are Quantized
| Quantized energy levels in hydrogen |
Bohr Atom Of Vanadium
The basic feature of quantum mechanics that is incorporated in the Bohr Modeland that is completely different from the analogous planetary model is that theenergy of the particles in the Bohr atom is restricted to certain discretevalues. One says that the energy is quantized. This means that onlycertain orbits with certain radii are allowed; orbits in between simply don'texist.The adjacent figure shows such quantized energy levels for the hydrogen atom.These levels are labeled by an integer n that is called a quantum number.The lowest energy state is generally termed the ground state. The stateswith successively more energy than the ground state are called the first excitedstate, the second excited state, and so on. Beyond an energycalled the ionization potential the single electron of the hydrogen atom is nolonger bound to the atom. Then the energy levels form a continuum. In the case ofhydrogen, this continuum starts at 13.6 eV above the ground state ('eV' standsfor 'electron-Volt', a common unit of energy in atomic physics).
Although this behavior may seem strange to our minds that are trainedfrom birth bywatchingphenomena in the macroscopic world, this is the way things behave in thestrange world of the quantum that holds sway at the atomic level.
Atomic Excitation and De-excitation
Atoms can make transitions between the orbits allowed by quantum mechanics byabsorbing or emitting exactly the energy difference between the orbits. Thefollowing figure shows an atomic excitation cause by absorption of a photon andan atomic de-excitation caused by emission of a photon.Bohr Atom Oxygen
| Excitation by absorption of light and de-excitation by emission of light |
In each case thewavelength of the emitted or absorbed light is exactly such that the photoncarries the energy difference between the two orbits. This energy may be calculated bydividing the product of the Planck constant and the speed of lighthc by the wavelength of the light). Thus, an atom can absorbor emit only certain discrete wavelengths (or equivalently, frequencies orenergies).
